
Accentrix injection is one of the anti-VEGF injections available for use. Antivegf eye injections are used in certain eye conditions, mainly retinal diseases. One can read more about eye injections. A few other anti-VEGF drugs are available, and you can read more about Avastin and Eyelea. There is also another drug that is used in similar conditions called Ozurdex. This is a sustained-release steroid injection.
Ranibizumab is the drug present in accentrx anti VEGF injection. It is sold by Novartis under the brand name of Accentrix in India. Intravitreal ranibizumab injection was first approved by the FDA in 2006 for wet age-related macular degeneration. Since then it has been approved for the treatment of macular edema following retinal vein occlusion and diabetic macular edema. Most recently, it was approved in 2015 for patients with diabetic retinopathy.
Accentrix and Lucentis are both manufactured by Novartis pharmaceuticals. The name of the drug is ranibizumab. The brand name was known as Lucentis when it was first launched. Currently, in India, it is known as Accentrix. So, there is no difference between the two injections.
As mentioned earlier there are a few retinal conditions where the Accentrix injection is used. You can read more about ARMD, diabetic eye disease. Some other conditions are retinal vein occlusions.
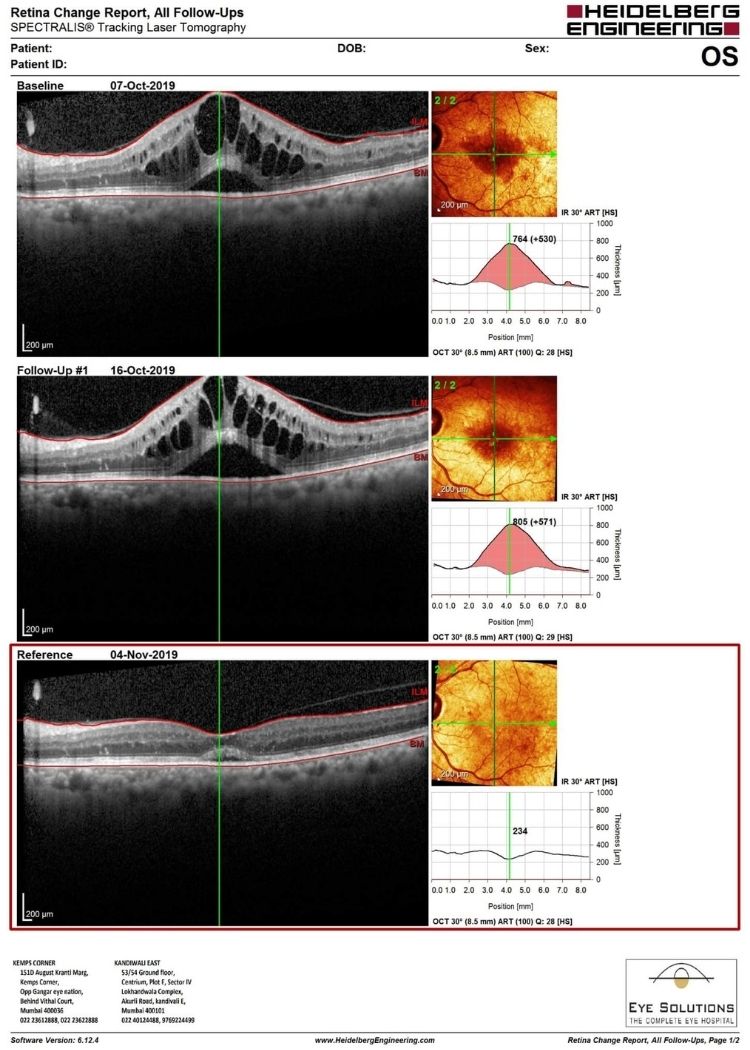
Accentrix is a recombinant, humanized immunoglobulin antibody fragment developed for intraocular use. Ranibizumab binds to the receptor-binding site on VEGF-a, which inhibits the binding of VEGF molecules to their receptors on the surface of endothelial cells. Ranibizumab blocks all isoforms of VEGF-a. It basically prevents the VEGF molecule from having its desired effect. This is how Accentrix reduces macular edema.
Macular edema is swelling or collection of fluid in the central part of the retina. The fluid collection occurs due to 2 main reasons :
Avastin has a drug called Bevacizumab. This drug was the first anti-VEGF used for the treatment of eye conditions. Though it hasn’t been FDA approved, off-label use continues for various ophthalmic conditions.
The active part of the molecule is similar in Bevacizumab and Ranibizumab. However, Bevacizumab is the whole anti-VEGF antibody (150 kd), while Ranibizumab is an antibody fragment. Bevacizumab has a longer half-life in the systemic circulation, while Ranibizumab is believed to penetrate the retina better and has a higher affinity to VEGF-a than Bevacizumab. In other words, accentrix injection has better retinal penetration than Avastin.
These differences could impact the safety and efficacy of these drugs.
Eylea is an anti-VEGF drug known as Aflibercept or VEGF trap eye. It blocks all the isoforms of VEGF, has a greater binding affinity than Ranibizumab, and has a longer duration of action.
The improved pharmacokinetics of Aflibercept decreases the frequency of dosing in patients.
The Accentrix injection is available as a single-use vial, and 0.1 ml of the drug is present. The entire 0.1ml is injected into the eye intravitreally.
The general dosing schedule is to start with three injections one month apart, monitor the retinal swelling and vision, and decide on further injections. These three initial shots would be considered a fixed schedule. We also treat on a need to basis. The eye is imaged every month. Based on the findings of the oct scan, your doctor would treat or not treat.
A popular treatment protocol is the 'treat and extend' protocol. Here the first three injections are given monthly. After which, the duration between the injections is increased by two weeks from the fourth injection onwards.
As with any intraocular injection, there are some risks. The most commonly reported adverse reactions (>10%) included conjunctival hemorrhage, vitreous floaters, vitreous detachment, increased intraocular pressure, and eye pain. These occasionally occur with any intravitreal injection.
Severe complications are rare and include:
It is rare to lose vision as a result of an intravitreal injection. But if you do develop any of these red flag signs, we recommend that you call our clinic immediately.
Because both Accentrix and Lucentis injections are given in the eye, it becomes important to maintain strict aseptic protocols. In other words, these eye injections are given in the operation theatre and not in the outpatient clinic.
The patient is taken to the OT and made comfortable in the OT bed. The eye to be injected and the surrounding area is cleaned with an antiseptic, and the area is covered with sterile plastic drapes.
A wire speculum opens the eye, and eye drops numb the eye. After the eye injection, the eye is patched with some antibiotic eye ointment.
The patient removes the patch after two hours and starts antibiotic eye drops six times a day for a week. The patient is also asked not to take a head bath for a day after the injection. Usually, your doctor will again see the patient after a week or two.
As mentioned earlier, we inject the eye in the operation theatre, and thus there is a cost involved in administering this injection. The Accentrix injection rate in India is 21000, and eye solutions chargers 13000 as charges to give the injection. Thus your total Accentrix injection price in India would be around Rs 34000 – 35000.
Please note that this price does not equate to any anti-VEGF injection price in India. Each Antivegf injection has a different price. Only the hospital charges to administer the injection remain constant. The cost of the anti-VEGF varies.
Two pivotal trials determined the effectiveness of Ranibizumab ( Inj Accentrix ): the minimally classic/occult trial of the anti-VEGF antibody ranibizumab in the treatment of neovascular age-related macular degeneration (marina) and the anti-VEGF antibody for the treatment of predominantly classic choroidal neovascularization in age-related macular degeneration (anchor). Marina and anchor were the first phase 3 trials to improve visual outcomes for all forms of choroidal neovascularization in nvamd. Based on this evidence, Ranibizumab was approved by the FDA on June 30, 2006, to treat nvamd.